The unit cell completely describes the structure of the solid which can be regarded as an almost endless repetition of the unit cell. And Justice Science Chemistry Mathematics FinanceFoodFAQHealthHistoryPoliticsTravelTechnology Random Article Home FAQ How Many Atoms Simple Cubic Unit Cell FAQ How.

Pin By Charlie Bravo On Ingenierias Unit Cell The Unit Cell
The simple cubic unit cell is a cube all sides of the same length and all face perpendicular to each other with an atom at each corner of the unit cell.

. In addition each of the particles in the center of the face-centered cubic cell is shared by 2. The volume of the unit cell is readily calculated from its shape and dimensions. Simple cubic SC structures have one atom per unit cell and a 52 packing efficiency.
The Body-Centered Cubic BCC unit cell can be imagined as a cube with an atom on each corner and an atom in the cubes center. The same situation exists for the edge or corner particles in the face-centered and body-centered cubic forms. The corners contribute only one net atom and.
The simple cubic unit cell is a cube all sides of the same length and all face perpendicular to each other with an atom at each corner of the unit cell. Body-centered cubic BCC structures have. 41 72 How useful was.
The unit cell completely describes the structure of the solid which can be regarded as an almost endless repetition of the unit cell. If the atomic mass is known the mass of the unit cell is equal to atomic mass Avogadro number atomic mass 6022 10 23 g. Arrangements duplicate themselves every other layer.
It is one of the most common structures for metals. These are corner atoms so each one only contributes one eighth of an atom to the unit cell thus giving us only one net atom. Hence density of unit cell mass of unit cell volume of unit cell.
Simple Cubic Unit Cell is the least complex cubic unit cell and contains only 1 atom. Each layer is offset from the layer before. Each corner of the unit cell is defined by a lattice point at which an atom ion or molecule can be found in the crystal.
Each atom located on the corner contributes 18 th of the original. In the simple cubic cell each corner atom is shared by 8 differenent unit cells. 15 rows The two-dimensional arrangement of the spheres can be extended to the third dimension to form a.
The volume of the unit cell is readily calculated from its shape and. Simple Cubic Unit Cell. The simplest cubic lattice is shown in interactive 3D for advanced school chemistry and undergraduate chemistry education hosted by University of Liverpool.
19 rows Simple Cubic Unit Cell is the least complex cubic unit cell and contains only 1 atom. There are 8 eighths one in each corner for a total of ONE atom in the unit cell. BCC has 2 atoms per unit cell lattice constant a 4R3 Coordination number CN 8 and Atomic Packing Factor APF 68.
The simple cubic unit cell is the simplest repeating unit in a simple cubic structure. A simple cubic unit cell contains one atom. The simple cubic unit cell is delineated by eight atoms which mark the actual cube.
There are four types of cubic cell. Body centered Cubic Unit Cell- Total 2 atoms present in a body centred unit cell. A Simple Cubic Unit Cell There is only 1 atom in a simple cubic unit cell.
They vary in how the atomsspheres are arranged inside of it. This unit cell uses nine atoms eight of which are corner atoms forming the cube and one more in the center of the cube. They are called simple cubic face-centred cubic and body-centred cubic.
For a simple cubic unit cell 8 atoms are located on 8 corners of the lattice. The volume of the unit cell is 8 r 3. Home Inorganic Chemistry Solid state Cubic structures Simple cubic.
The face-centered cubic unit cell also starts with identical particles on.

Comparison Of Space Filling In Different Cubic Structures Unit Cell Material Science Chemistry Textbook

Pin By Charlie Bravo On Ingenierias Unit Cell Crystal System Lattice

Basic Crystal Concepts Bravais Lattice Unit Cell Crystal Lattice

Primitive And Non Primitive Unit Cell In 2022 Unit Cell The Unit Primitive

Types Of Unit Cell Unit Cell The Unit Cell

Unit Cell Face Centered Cubic Crystal Lattice Structures Physical Electronics

Ncert Solutions For Class 12 Chemistry Chapter 1 The Solid State Cbse Tuts Class12chemistrychapter1ncertsolutions Chemistry Class Solutions

Crystallographic Directions Physical Electronics

Face Centered Cubic Fcc A Closest Packing Structure Lattice Structure Crystalline Solid Chemistry

Cubic Lattice From Wolfram Mathworld Cell Forms Unit Cell Lattice

Planes In A Cubic Unit Cell Physical Electronics Unit Cell The Unit Physics
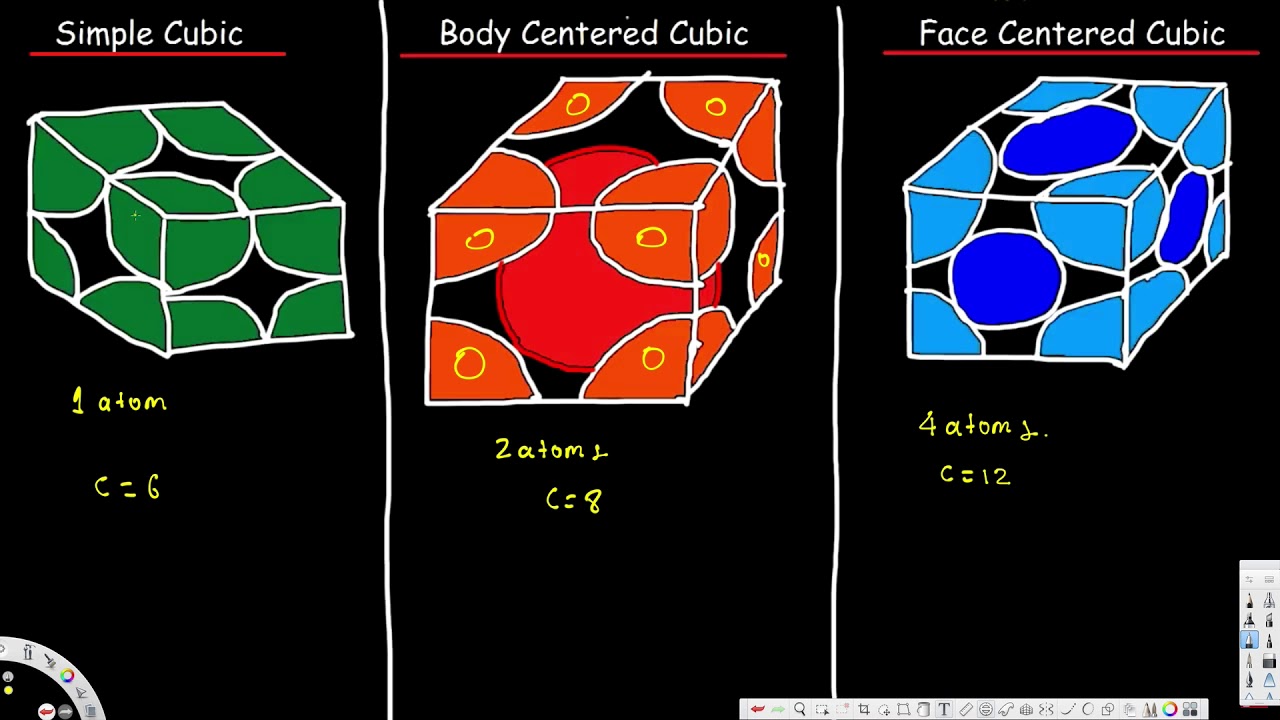
Unit Cell Simple Cubic Body Centered Cubic Face Centered Cubic Crystal Lattice Structures

Chemistry Liquids And Solids 32 Of 59 Crystal Structure Seven Types Of Unit Cells Chemistry Crystal Structure Unit Cell

Quantum Well Infinite Potential Well A 2 To A 2 Physical Electronnics Physics Wave Function Quantum

Unit Cell Simple Cubic Structure Physical Electronics

Pin By Flathorn On Sacred Geometry Bravais Lattice Chemistry Chemistry Education

Planar Packing Fraction Factor For The Body Centred Cubic 111 Plane

Unit Cell Body Centered Cubic Crystal Lattice Structures Physical Electronics

